Photocatalysis uses light to excite electrons to initiate chemical reactions, which can achieve the breaking and reorganization of chemical bonds under mild conditions. Compared with the traditional heating reaction, it has the advantages of green cleaning, safety and environmental protection and easy control. In recent years, photocatalytic reactions have continuously made breakthroughs in the field of synthetic chemistry. A series of photocatalytic reaction systems have been discovered and successfully applied to the synthesis of various complex compounds, demonstrating outstanding synthetic value and application potential. However, currently photocatalysts are mainly noble metal complexes (Ir, Ru, etc.) and organic dyes. The catalytic system absorbs visible light to excite electrons from the ground state to the excited state, and then undergoes single electron transfer (SET) with the substrate to achieve the catalytic cycle ( Picture 1-1). However, this visible light-induced intramolecular charge transfer requires a large π delocalized structure in the molecule or a metal-ligand complex conjugate to generate a band gap in order to have an absorption effect in the low-energy visible light range. Therefore, in order to realize visible light excited electrons The transition requires the introduction of a complex molecular structure, which inevitably increases the cost of the photocatalyst.
Light-induced charge transfer between molecules can occur between electron donors and acceptors through non-covalent bonds. It does not limit every substrate (donor or acceptor) to have an absorption effect in a specific wavelength range It only needs to satisfy that the complex formed by the combination of the donor and the acceptor has an absorption effect in a specific wavelength range, which can simplify the composition of the photocatalytic system and reduce the cost of the catalyst. Although this kind of light energy utilization method has been widely used in photovoltaic devices, the application of the mechanism of catalytic reduction and catalytic cycle to the field of synthesis is still a new concept that has not been proposed.
The research team of Fu Yao and Shang Rui from the University of Science and Technology of China has long been devoted to the research in the field of decarboxylation conversion of organic carboxylic acids derived from biomass. Based on the concept of green catalysis, the team first proposed a new concept of intermolecular charge transfer based on visible light excitation for photo redox catalysis, and found a simple and easily available, highly efficient and environmentally friendly non-metallic anion complex photocatalytic system, which was successful The decarboxylation coupling reaction under mild conditions is achieved, which breaks through the limitation that traditional reactions require noble metal photocatalysts or organic dyes. The research result is titled "Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide", and it was published online in the form of a long article in the international authoritative journal "Science" on March 29, 2019.
In this paper, through theoretical calculations, it is found that the release energy value of sodium iodide, triphenylphosphine and active carboxylic acid ester to form charge transfer complex (CTC) by Coulomb force is 3.8 kcal / mol. According to Marcus theory, the electron transfer energy barrier of iodine to phthalimide fragments is 61.2 kcal / mol, and without adding triphenylphosphine, a similar electron transfer process must overcome a higher energy barrier (86.5 kcal / mol). On the one hand, triphenylphosphine can promote the transfer of electrons, on the other hand, it can trap iodine radicals to form Ph3P-I •. Theoretical calculation results show that Ph3P–I • has reducing ability, and its spin density is delocalized between the iodine atom and the phosphine atom. This anionic complex is similar to the oxidation state of the redox photocatalyst and has the role of participating in the construction of light induction The possibility of a redox cycle (Figure 1-2).
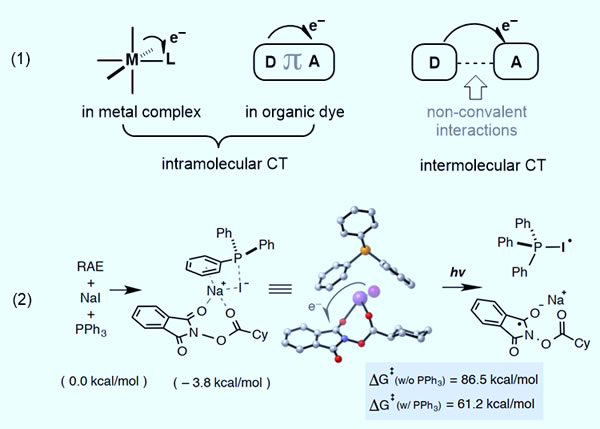

Figure 1. Photocatalyst system and charge energy transfer model, photocatalytic reaction device
Combined with the above theoretical calculation research, the research team successfully realized the catalytic decarboxylation reaction of fatty carboxylic acid derivatives. The generated alkyl radical intermediates can be combined with a variety of substrates to achieve the Minisic reaction and Heck reaction under mild conditions. Through this catalytic system, a variety of natural and unnatural amino acids can react with the enol silicon ether, and when it is scaled up to the gram scale, it can still maintain a high catalytic efficiency, providing an effective method for the preparation of β-aminoketone compounds way. More valuable is that when the catalytic system is co-catalyzed with commercial chiral phosphoric acid, the amino acid can react with the nitrogen heterocycle to realize the asymmetric α-amino alkylation of the C2 position of the nitrogen heterocycle, which is a nitrogen-containing heterocyclic The asymmetric modification of drug molecules provides an effective means. In addition, the alkylamine derivatives widely present in natural products and synthetic chemicals can also undergo deamination Heck reaction. (figure 2).
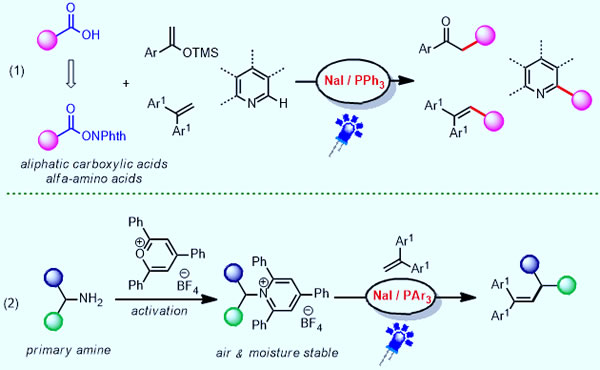
Figure 2. Light-induced coupling reaction of decarboxylation and deamination catalyzed by nonmetallic anion complex
This new type of non-metallic anion complex photocatalytic system greatly reduces the cost of catalysts and can be applied to the synthesis of a variety of important functional molecules. It solves the problems of transition metal residues in the synthesis of functional compounds and drugs. It is a biomass carboxylic acid molecule. Transformation, chiral drug synthesis and peptide modification provide new means, with important synthetic chemical value and good industrial application prospects.
The research work was supported by the Ministry of Science and Technology, the Fund Committee, the Chinese Academy of Sciences and the Hefei University Science Center.
B18.16.6 Nylon Insert Lock Nut
Chuangtuo Jinggong (Jiangsu) Co., LTD , https://www.chtofastener.com