In recent years, the production of hydrogen by electrolyzed water has received widespread attention, and finding cheap and efficient electrocatalysts that can replace precious metals has become a hot research topic. Graphene has attracted extensive attention from researchers due to its advantages of good conductivity, excellent chemical stability and easy chemical modification, and people are committed to developing it as a highly active catalyst for hydrogen production from electrolyzed water. Existing research results show that the doping of nitrogen and other heteroatoms can regulate the electronic structure of the carbon atoms adjacent to the heteroatoms, enhance the adsorption of the active sites of the carbon atoms and the reaction intermediates, and thereby improve the electrocatalytic hydrogen evolution of carbon-based materials such as graphene Performance, however, the traditional pyridine, pyrrole and graphite nitrogen doping modes have poor control over the performance of graphene and other carbon-based catalysts. There is still a big gap compared with the reported high-activity metal-based catalysts. Researchers at the University of Science and Technology of China have revealed through density functional theory calculations (DFT) that bi-graphite nitrogen doping within a six-membered ring of graphene lattice can significantly change the carbon atoms in the material (the carbon atoms combined with two nitrogen atoms) ) Electronic structure, reducing the ΔGH * value of the carbon active site to very close to 0 eV, is expected to further improve the hydrogen evolution catalytic activity of carbon-based materials. In this study, the metal-organic framework compound Cu-BTC was used as a precursor, and graphene-like particle aggregates were obtained through calcination and solvent heat treatment. After CV cycle, its acidic electrocatalytic hydrogen evolution performance gradually improved to reach the optimal value at 10 mA. At a current density of / cm2, the overpotential is only 57 mV, and the Tafel slope is 44.6 mV / dec, showing the performance of electrocatalytic hydrogen evolution comparable to the reported highly active metal-based catalysts and Pt / C catalysts. Infrared spectroscopy, X-ray photoelectron spectroscopy, X-ray near-edge absorption fine structure, and solid-state nuclear magnetic resonance characterization results show that the carbon-based material has formed a new structure of a double-graphite nitrogen doped in a graphene lattice six-membered ring, Two adjacent graphite-type nitrogen-bonded carbon atoms are catalytically active sites. This bonding method is conducive to enhancing the adsorption of H on the C-active site, thereby enhancing catalytic activity.
The research results are published online in the international journal "Angel. Chem. Int. Ed" under the title of Dual Graphitic-N Doping in a Six-Membered C-Ring of Graphene-Analogous Particles Enables an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. . DOI: 10.1002 / anie.201908210).
Chen Qianwang, a professor at the National Research Center for Microscale Physical Sciences and the School of Chemistry and Materials Science, Hefei, Chinese University of Science and Technology, is the corresponding author of the thesis. Lin Zhiyu, a PhD student at the Chinese University of Science and Technology, and Yang Yang, the postdoctoral fellow, are the co-first authors. Hefei Synchrotron Radiation National Laboratory, Shanghai Synchrotron Radiation Light Source and Hefei Steady State Strong Magnetic Field Center have provided important assistance for the characterization and analysis of the experimental results. The research was supported by the National Natural Science Foundation of China.
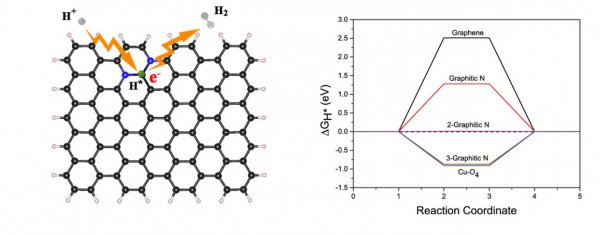
Schematic diagram of the electrocatalytic hydrogen evolution reaction of bigraphite nitrogen doped in a graphene lattice six-membered ring structure and Gibbs free energy calculation results [black spheres represent carbon atoms, blue spheres represent nitrogen atoms, and two The carbon atoms connected by two graphite-type nitrogen atoms are the active sites for electrocatalytic hydrogen evolution. The related Gibbs free energy calculation results give the structure of two graphite-type nitrogen doped in a graphene lattice six-membered ring (2-Graphitic N) ΔGH * is 0.01eV.]
Allen Socket Screw,Allen Head Cap Screw,Socket Head Cap Screws,Stainless Steel Socket Head Cap Screws
Taizhou Fengye Metal Products Co., Ltd. , https://www.fyhandware.com