Scientists claim to have developed an inexpensive device that can use light to break water into oxygen and clean energy hydrogen. The body of the hydrolyser is a silicon semiconductor wrapped with an ultra-thin nickel layer. The author of the article said that the device can be used for the large-scale industrial production of clean energy hydrogen. This article was published in the latest issue of Science. The main purpose of this study was to use hydrogen energy batteries instead of generating electricity when the solar cells were not working (no sunlight or weak light intensity).
"Solar cells can only work when there is a sun." Co-author Hongjie Dai from Stanford University said in an interview: "In the absence of the sun, the equipment can only use the electricity generated by the power plant using traditional coal or wind energy." Now We provide a greener solution where we can use a hydrogen battery to supply electricity at night, on cloudy days, or when electricity is too high.
How to break down water
In order to provide clean hydrogen to fuel cells, scientists turned to an emerging technology - photocatalytic water decomposition. The two semiconductor electrodes communicate with each other at both ends of the water tank. In normal conditions, the electrode absorbs light energy and uses it to decompose water into hydrogen and oxygen. Oxygen is released directly into the air. When people need energy, this process will be reversed. The hydrogen stored in the air and the oxygen in the air will form an energy battery to generate electricity and produce pure water. The whole process is sustainable and does not release greenhouse gases. However, finding a cheap water decomposing equipment has always been a challenge. Scientists have been searching for an inexpensive material to make a hydrolyzer and have been used for practical production.
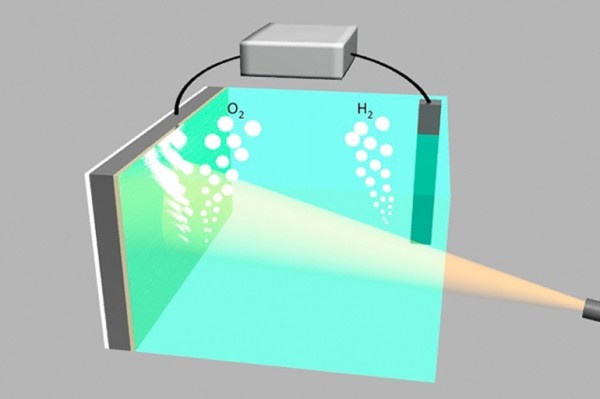
Silicon material
Stanford University graduate student Michael J. Kenney, co-author of this article published in Science, said in an interview: "Silicon material has been widely used in solar cells, and it is also the most suitable inexpensive material, but silicon once Degradation occurs when the electrolyte solution contacts."
In 2011, Stanford's research team also added an ultra-thin layer of titanium dioxide and tantalum to the silicon material to overcome this defect in silicon. The corrugated silicon material worked continuously for 8 hours without corrosion.
"This is indeed an exciting result, but it needs to be used for long enough stability in actual production." Dai said, "And the price of precious metal cerium is also very high. We can find an inexpensive metal catalyst instead." More suitable."
In order to find an inexpensive alternative metal, Dai suggested that Kenney and his friends try to coat the silicon electrode with a normal nickel coating.
"Nickel is quite corrosion-resistant," Kenney said. "And nickel is also a catalyst for a more active oxygen production reaction. Plus, there is a lot of nickel on Earth. All of these conditions make nickel very competitive. â€
Nickel nano-thin layer
In the experiment, Dai's research team coated the electrodes with a 2 nm thick layer and placed them in potassium borate solution. When the light was turned on or the power was turned on, the electrode began to water at the working point and no corrosion was found for 24 hours of continuous work. To provide battery performance, the researchers added a little lithium to the solution. After lithium was added, there was no evidence of any corrosion on the surface of the battery after 80 hours of continuous operation.
Compared with previous experiments, this result has made great progress. Dai said: "Our laboratory has developed a long-lasting silicon photoelectrode. The experimental results show that the ultra-thin nickel layer not only allows the electrode to resist corrosion but also acts as a catalyst, greatly increasing the rate of water decomposition." "Fun Thomas Edison once said that adding a small amount of lithium to the electrolyte can make nickel batteries perform better. After many years of excitement, we found that this can also make electrolyzed water installations perform better."
soap rack,soap holder,soap stand,stainless steel soap holder,steel wire soap stand,etc.Let your bathroom become more simple and upscale!Applicable to families, hotels, home stay and other places to use.
304 stainless steel never rust, will easy to clear, it's also very durable!
we are 15 year factory, we had big engineer team, and strong production line, can give you good serve and quanlity. Welcome to cooperation!
Soap Rack,Soap Holder,Soap Stand,Stainless Steel Soap Holder,Stainless Steel Wire Soap Stand
Shenzhen Lanejoy Technology Co.,LTD , https://www.copper-nut.com